You are here
New Hope for Malaria Prevention in At-Risk Children
Every 75 seconds, roughly the time it takes to drink a glass of water or return an email, a child under the age of five dies of malaria. The toll is especially high in sub-Saharan Africa—a region where 96 percent of the world’s malaria deaths occur. Because their immune systems are not yet fully developed, a staggering 80 percent of those dying are children.
Despite these grim statistics, there is hope.
Malaria, which is caused by parasites spread by infected mosquitos, is both preventable and treatable. In recent years, effective malaria prevention strategies like insecticide treated nets and indoor residual spraying (IRS) have significantly reduced the number of malaria cases and malaria-related deaths in Africa.
In 2021, the World Health Organization also recommended a malaria vaccine called RTS,S that could save the lives of tens of thousands of children each year. Though the vaccine represents a huge step forward in the fight against this deadly disease, it currently requires a complex, four-dose regimen that is only approximately 40% effective over four years. While researchers look for ways to increase the impact and efficiency of malaria vaccines, it is critical that we continue to build on this important progress by developing new strategies to protect at-risk children.
In June 2022, the CDC Foundation partnered with the Centers for Disease Control and Prevention (CDC), the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH) and the Bill & Melinda Gates Foundation to develop a new clinical trial with the Kenya Medical Research Institute (KEMRI) and the Liverpool School of Tropical Medicine (LSTM) in western Kenya. The study will evaluate the use of a promising new malaria prevention tool—a monoclonal antibody known as L9LS.
Monoclonal antibodies (mAbs) are natural immune proteins that are isolated from humans following vaccination or infection and then produced in larger quantities in a laboratory. Monoclonal antibodies have been developed and safely used against a number of infectious diseases. Like naturally occurring antibodies, they recognize infectious bacteria, viruses and parasites in the body and target them for destruction by white blood cells.
The L9LS monoclonal antibody prevents malaria by neutralizing the parasites in the body before they can infect the liver cells. In 2021, trials conducted by the Vaccine Research Center at the NIH, which initially identified and developed the mAb, showed that L9LS was safe and highly effective at preventing infection in American adults exposed to malaria under controlled conditions. Importantly, L9LS was effective at low doses and with just one injection under the skin—making this a potentially cost-effective approach for malaria prevention that could be used in people of all ages.
“With malaria vaccines, the immune system is triggered in a complex process to create a number of different antibodies,” said Laura Steinhardt, MPH, PhD, epidemiologist at CDC’s Division of Parasitic Diseases and Malaria. “But by injecting a monoclonal antibody, you are not depending on the body’s response but are directly giving high doses of one of the most effective antibodies to target the malaria parasites and prevent infection.”
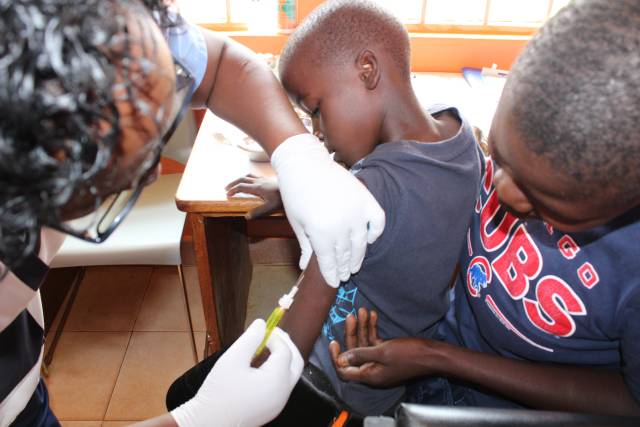
The L9LS trial will build on NIH’s work by evaluating the safety and tolerability of a one-time injection of L9LS in healthy Kenyan children ages 5 months to 10 years. It will also assess the impact of one or two doses of L9LS in protecting younger children, ages 5 months to 59 months, in areas where malaria transmission is high.
The first phase of the trial began on October 1, 2022, with the initial doses of L9LS administered to participating children ages 5-10 years. The children will be monitored closely for any side effects or adverse reactions over the next three months.
In addition to Dr. Steinhardt, Titus Kwambai, MD, PhD, epidemiologist with CDC and Feiko ter Kuile MD, PhD, professor of tropical epidemiology at LSTM were on site in Kenya for the launch of this trial that, if effective, could help prevent the spread of a disease that has threatened mankind for thousands of years.
“This is an exciting strategy and a crucial moment in the fight against malaria,” Dr. Kwambai said. “This study will give us valuable information about the safety and efficacy of monoclonal antibodies, which may prove to be an intervention that can protect children from this deadly disease and accelerate progress toward eliminating malaria.”
Photo credit: Antony Muyeshi / CDC Foundation